DOES THE FREQUENT REUSE OF COOKING OIL ELEVATE CANCER RISK?
Vegetable oil is valuable for the vitamins, antioxidants, and energy it provides. It also functions as a transporter of several vitamins to body cells (Kritchevsky, 2008). The use of vegetable oil is common in food preparation. Deep frying is a popular method in which food is immersed in hot oil at temperatures ranging from 140–200 °C.
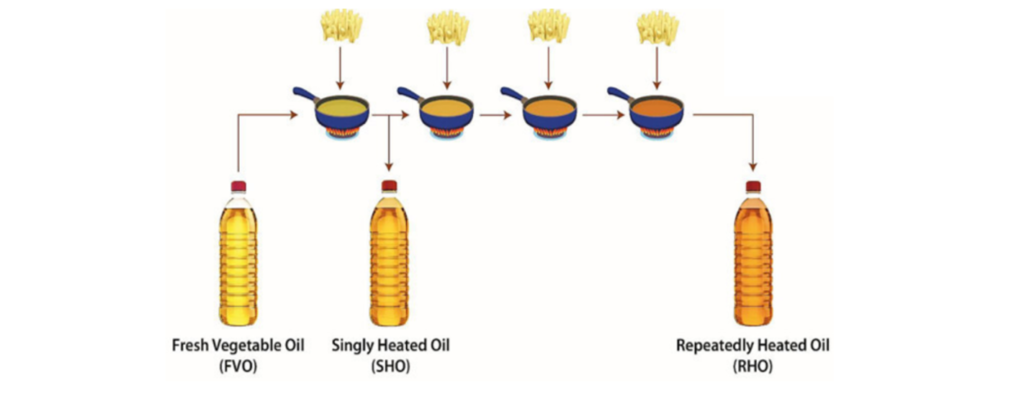
Figure 1: Changing color of the cooking oil
During deep frying, simultaneous heat and mass transfer enable the desirable qualities of fried food to be achieved. However, during deep frying, a number of changes such as oxidation, hydrolysis, isomerization, and polymerization occur, which alter the flavor and spoil the compounds present in cooking oil. These changes affect both the quality of the oil and the fried food.
THE CHANGES IN COOKING OIL DURING DEEP FRYING
Deep frying causes changes in the physicochemical, nutritional, and sensory properties of the oil. Food with varying proximate compositions, such as vegetables, chicken, potatoes, and fish, tends to transfer various substances like fat, water, salt, sugar, and antioxidants into the frying oil, altering the rate and type of reactions that occur in the oil [1, 2].
According to Good [4], using vegetable oil for deep frying repeatedly reduces the smoke point, causing the oil to smoke even at lower temperatures. Due to the harmful substances present in both the oil and the fried food, degraded frying oil may have a negative impact on human health [5].
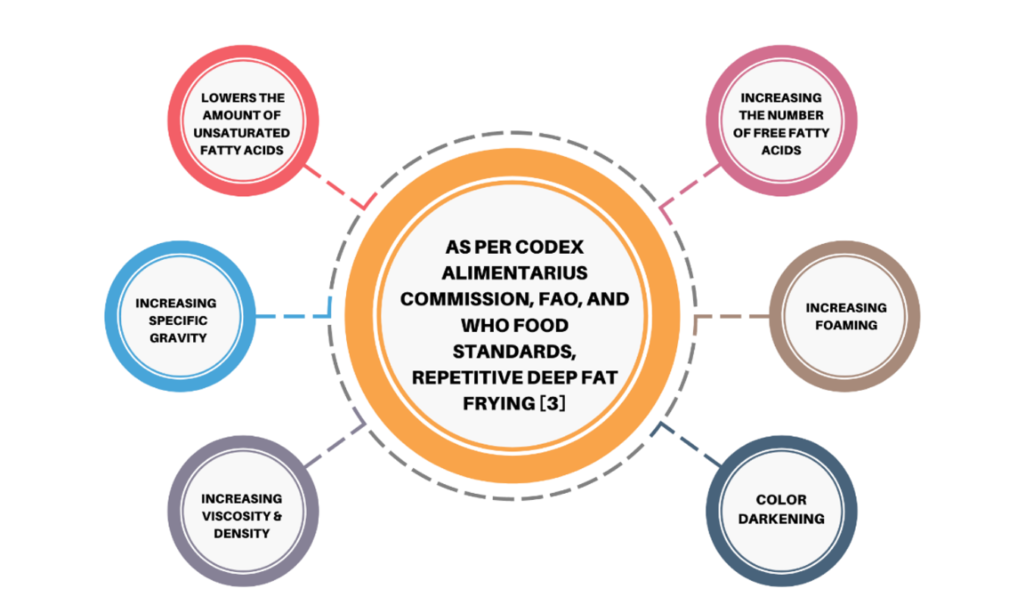
USE OF RCO IN SOUTH ASIAN COUNTRIES
In the South Asian region, various food items are cooked by deep frying, including Kachori, Poori, Wada, Samosa, Bread Pakoda, French Fries, Patties, and more. The physicochemical and antioxidant qualities of cooking oil used by local fried food vendors change with repeated heating, and their consumption increases the risk of various diseases such as atherosclerosis, cardiovascular disease, diabetes, and cancer.
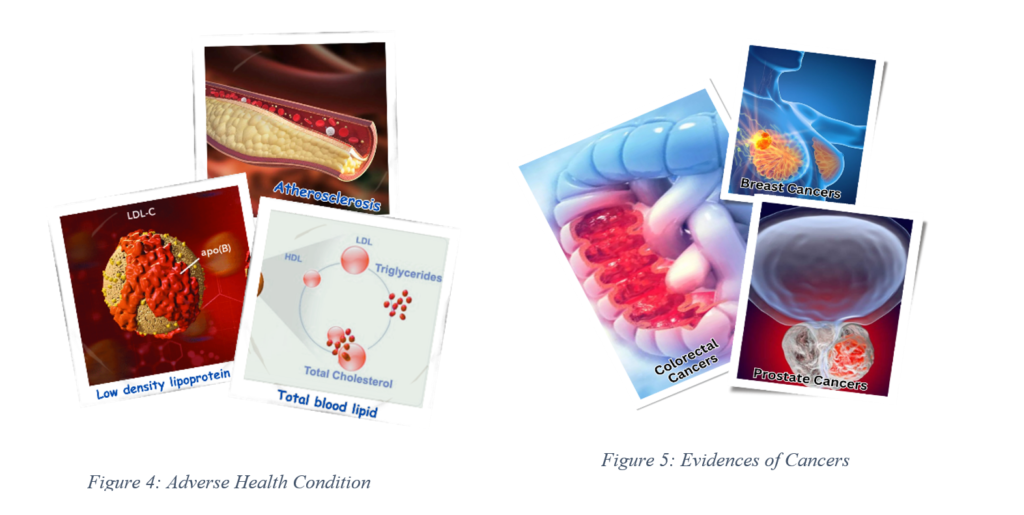
Repeatedly heated oil can generate compounds such as polycyclic aromatic hydrocarbons (PAHs), which are known to be carcinogenic. PAHs are produced during the combustion of organic materials and are harmful when consumed or inhaled. Studies have shown that the consumption of RCO and inhalation of cooking fumes are associated with an increased incidence of genotoxic, mutagenic, and tumorogenic effects, leading to an elevated risk of various cancers, including lung, colorectal, and prostate cancer [6].
Research results revealed that almost all repeated frying oils collected were above the permissible limit for Free Fatty Acid, Iodine Value, and Peroxide Value. It was noted that the highest values were obtained in the 10th batch of repeated frying oils. Increased usage of the repeated frying oils also resulted in a decrease in total phenolic content and total flavonoid content compared to values obtained from fresh oil samples [7].

Figure 3: Deep Frying Foods Prepared by using RCO
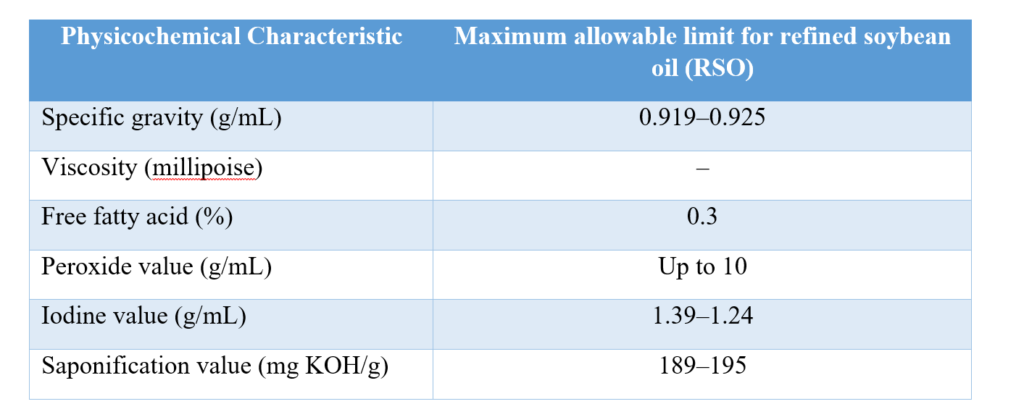
Source: Codex Standard for Named Vegetable Oils (CODEX-STAN 210-1999)
EFFECT OF REHEATED VEGETABLE OILS ON ANTIOXIDANT ACTIVITY
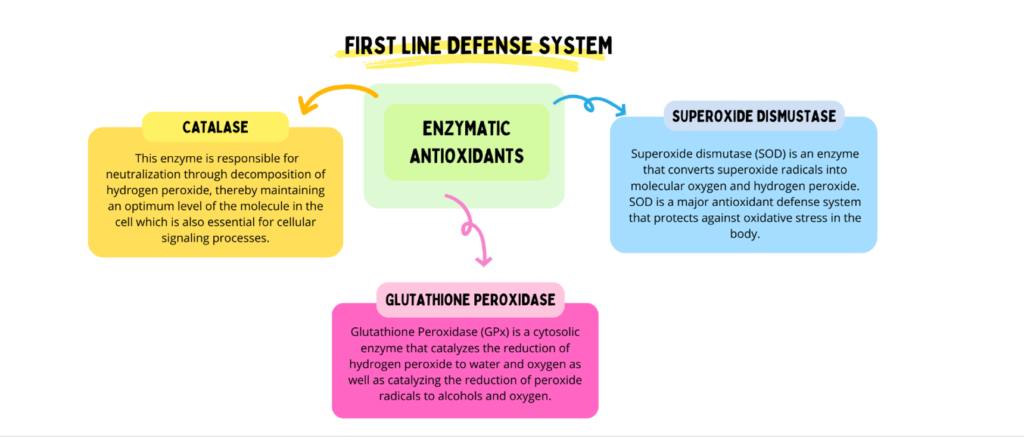
Excessive generation of reactive oxygen species (ROS), coupled with a reduced availability of antioxidants, predisposes cells to a state of oxidative stress. ROS are highly reactive and unstable in nature. Antioxidants present in oil inhibit oxidative deterioration during the frying process and scavenge free radicals and ROS. Increasing the heating temperature and duration may alter the antioxidant activity in vegetable oils [8]. Enzymatic and non-enzymatic antioxidants maintain the balance of ROS levels and repair oxidative cellular damage. Enzymatic antioxidants are directly involved in the neutralization of ROS and are considered the first line of defense [9, 10].
Vitamin E consists of tocopherols and tocotrienols isomers, which are the major antioxidants in vegetable oils [11]. On the other hand, the second line of defense is represented by non-enzymatic radical scavenging antioxidants, which inhibit the initiation of the oxidation chain and prevent chain propagation [12, 13].
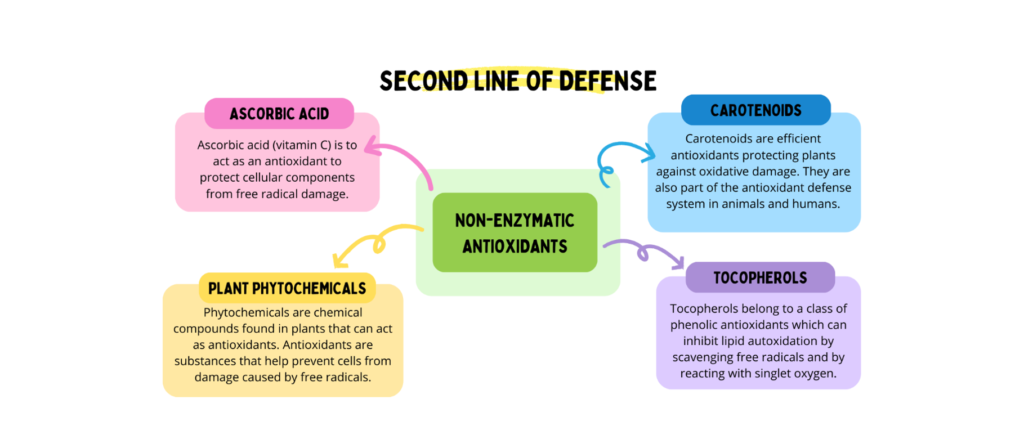
Natural polyphenols include phenolic acids and flavonoids [14]. These antioxidants protect cells and bio macromolecules against the harmful effects of free radicals and prevent oxidative degradation.
However, reheating vegetable oil at high temperatures leads to oxidation, which produces rancid odors and flavors [15, 16].
Subsequently, the oxidation process reduces both the nutritional value and the safety of fried food products through the formation of secondary products due to the peroxidation of polyunsaturated fatty acids (PUFAs) [17, 18].
The extent of oil degradation is measured by the peroxide index, which evaluates the amount of peroxides formed in vegetable oil during the oxidation process. The extent of oxidation rancidity is influenced by the number of frying episodes. A higher peroxide value indicates lower chemical stability of the oil.
In addition, soybean oil is high in PUFA content compared to palm oil, which has approximately a 1:1 ratio of saturated and unsaturated fatty acids with lower PUFA levels. Hence, soybean oil is more prone to oxidation than palm oil following repeated heating [19].
EFFECT OF REHEATED VEGETABLE OILS ON LIPID PEROXIDATION
Excessive free radicals cause alterations in the redox state of the human body, leading to lipid peroxidation. Oxidative damage to lipid structures can ultimately result in the disorganization and dysfunction of, as well as damage to, membranes, enzymes, and proteins [20].
Subsequently, lipid peroxidation impairs membrane functions, inactivates membrane-bound receptors or enzymes, and disturbs ion permeability and fluidity, which eventually leads to membrane rupture [22].
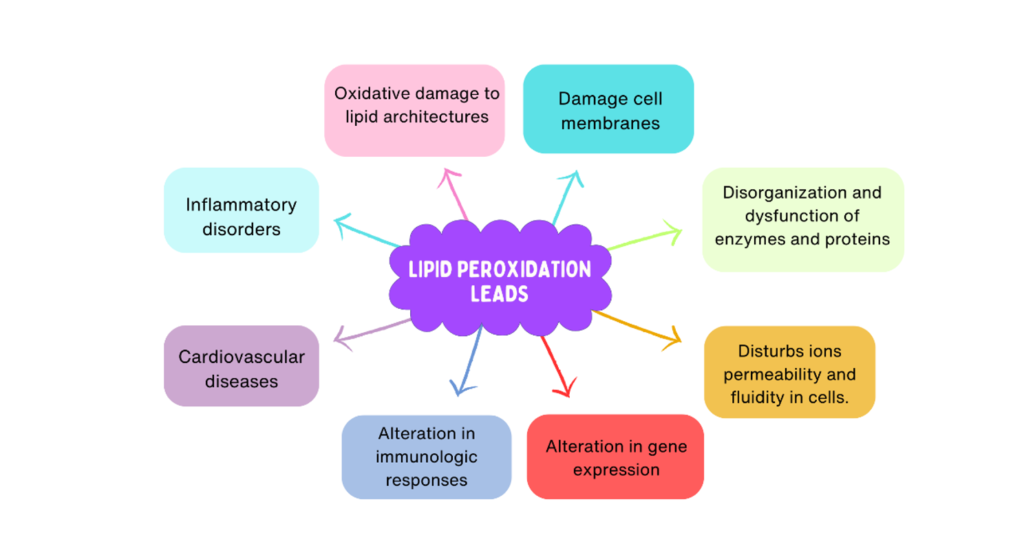
Moreover, reactive electrophilic end products of lipid peroxidation reactions, namely α- and β-aldehydes, are also detrimental to cell viability [21]. Lipid peroxidation provokes alterations in gene expression and immunologic responses [22]. Oxidative damage may accumulate over time, thereby contributing to cell injury and pathologies, including cardiovascular diseases [23, 24] and inflammatory disorders [25, 26].
SCIENTIFIC EVIDENCE OF POTENTIAL HEALTH EFFECTS BY USING REPEATED COOKING OIL
Several studies support the connection between reused cooking oil and cancer. For example:
- Kaleem et al. [28] found that poor-quality oil can be identified by changes in color, increased viscosity, lower smoke point, and high foam formation.
- Farag et al. [39] demonstrated that prolonged use of reheated oil caused organ damage, including kidney, liver, and heart damage, in animal models.
- Kritchevsky [30] reported that consuming oxidized fats increased LDL cholesterol and oxidative stress, both of which are risk factors for cancer.
STRATEGIES FOR DEALING WITH CARCINOGENS IN FOOD
Avoiding the repeated use of cooking oil is a good practice to maintain both the quality and safety of the oil. Reusing oil multiple times can lead to the formation of harmful compounds and the degradation of its nutritional value. Here are some recommendations to minimize the use of repeated cooking oil.

CONCLUSION
The frequent reuse of cooking oil, especially in deep frying, poses serious health risks due to the formation of harmful compounds such as PAHs and peroxides. Scientific evidence links the consumption of reused cooking oil to an increased risk of cancer, cardiovascular diseases, and other health problems.
By adopting safer cooking practices such as limiting the reuse of oil, using oils with high smoke points, and opting for healthier cooking methods we can reduce these risks. Prioritizing fresh oil and proper storage can make a significant difference in maintaining both the quality of your food and your health.
References
- Gómez A, Fregapane G, Salvador M, Gordon M. Changes in phenolic composition and antioxidant activity of virgin olive oil during frying. J Agric Food Chem. 2003. https://doi.org/10.1021/jf025932w.
- Juárez MD, Osawa CC, Acuña ME, Sammán N, Gonçalves LA. Degradation in soybean oil, sunflower oil and partially hydrogenated fats after food frying, monitored by conventional and unconventional methods. Food Control. 2011. https://doi.org/10.1016/j.foodcont.2011.05.004.
- Codex Alimentarius Commission/FAO/WHO Food Standards, Codex standards for named vegetable oils: CODEX STAN 210. CXSTAN 210-1999. 2000.
- Good J. Healthiest cooking oil chart with smoke points, baseline of health foundation. 2012. https://www.jonbarron.org/article/healthiest-cooking-oil-chartsmokepoints.
- Tena N, Aparicio R, Garcia-Gonzalez D. Thermal deterioration of virgin olive oil monitored by ATR-FTIR analysis of trans content. J Agric Food Chem. 2009. https://doi.org/10.1021/jf9012828.
- https://pubmed.ncbi.nlm.nih.gov/28925728/
- https://link.springer.com/article/10.1007/s44187-023-00046-8#:~:text=Repeatedly%20used%20frying%20oil%20causes,19%2C20%2C21%5D.
- Choe E, Min DB. Chemistry of deep-fat frying oils. J Food Sci. 2007; 72: R77-86.
https://pubmed.ncbi.nlm.nih.gov/17995742/
- Devasagayam TP, Tilak JC, Boloor KK, Sane KS, Ghaskadbi SS, Lele RD. Free radicals and antioxidants in human health: current status and future prospects. J Assoc Physicians India. 2004; 52: 794-804.
https://pubmed.ncbi.nlm.nih.gov/15909857/
- Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 2010; 4: 118-126.
https://pubmed.ncbi.nlm.nih.gov/22228951/
- Sundram K, Sambanthamurthi R, Tan YA. Palm fruit chemistry and nutrition. Asia Pac J Clin Nutr. 2003; 12: 355-362.
https://pubmed.ncbi.nlm.nih.gov/14506001/
- Rahman K. Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging. 2007; 2: 219-236.
https://pubmed.ncbi.nlm.nih.gov/18044138/
- Craft BD, Kerrihard AL, Amarowicz R, Pegg RB. Phenol-based antioxidants and the in vitro methods used for their assessment. Compr Rev Food Sci Food Safety. 2012; 11: 148-173.
https://ift.onlinelibrary.wiley.com/doi/abs/10.1111/j.1541-4337.2011.00173.x


